Cancer patients receiving anthracycline chemotherapy have more than twice the risk of developing heart failure compared to their peers without cancer. The ground-breaking RESILIENCE project aims to prevent heart failure in patients who need anthracyclines to treat their cancer.
International experts and patients from the project gathered on October 19th at the European Heart House, home of the European Society of Cardiology (ESC), as a unique think tank to improve the healthcare and quality of life for patients with cancer.
It is estimated that four million Europeans are diagnosed with cancer every year. Anthracycline chemotherapy has a prominent role in treating many forms of cancer – for example, up to 70% of patients with lymphoma receive an anthracycline regimen. Currently, there is no therapy to prevent anthracycline cardiotoxicity.
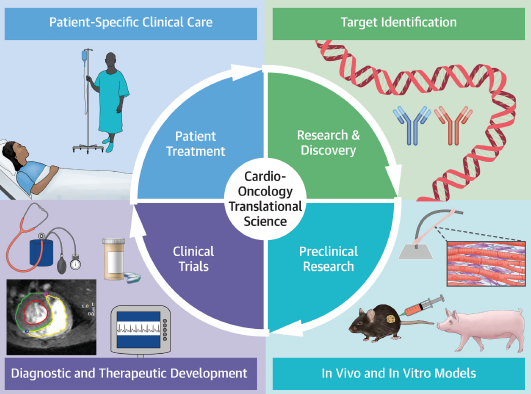
The multinational, EU-funded RESILIENCE project is testing a promising preventive treatment, called remote ischaemic conditioning, in lymphoma patients at risk for anthracycline cardiotoxicity. This is the first randomised trial to evaluate the intervention in patients with cancer receiving anthracycline chemotherapy. “If the study is positive, heart failure could be massively reduced in cancer survivors,” said project coordinator Professor Borja Ibáñez.
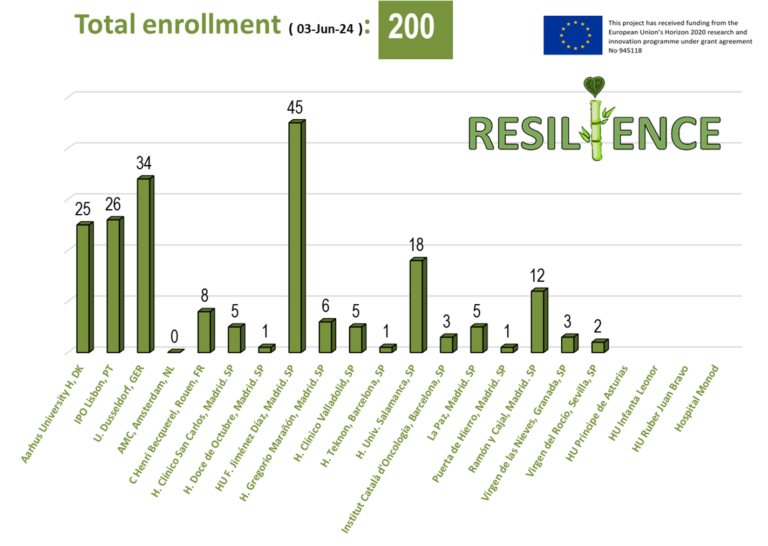
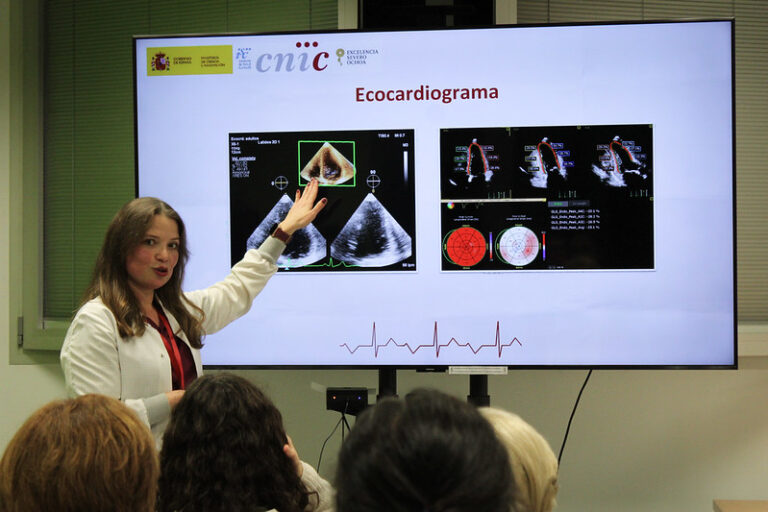
More than 600 patients with lymphoma undergoing anthracycline chemotherapy will be included from seven EU countries. Some 100 patients have already been enrolled. Participants are being randomly allocated to perform remote ischaemic conditioning or a sham intervention once a week, at home, during four months of chemotherapy. Remote ischaemic conditioning involves repeatedly stopping blood supply to one arm for five minutes at a time by inflating a blood pressure cuff. Substances released in the arm travel to the organs, making them resistant to injury. Experimental studies have shown that the treatment can reduce anthracycline-induced cardiotoxicity. State-of-the art imaging methods will be used to compare heart function between the two treatment arms.
The meeting brought together the project’s multidisciplinary consortium, which includes cardiologists, haematologists, oncologists, cardiac imagers, industry, policymakers, and most importantly, patients and patient associations. “The perspective of patients is essential in our efforts to reduce the heart damage caused by cancer treatment,” said Professor Teresa López-Fernández, principal investigator of RESILENCE and chair-elect of the ESC Council of Cardio-Oncology. “Patients have played a central role in designing the RESILIENCE trial and we will collect information on their experiences of treatment during the study.”
The first ESC Guidelines on cardio-oncology were published last year, and were accompanied by Guidelines written for patients. “The ESC is a key partner in the project,” added Professor Riccardo Asteggiano, principal investigator of RESILENCE and a past chairperson of the ESC Council of Cardio-Oncology. “In addition to hosting the October meeting, the ESC has contributed to the design of the research protocol, will play a key role in disseminating the results, and will help to educate cardiologists and cancer specialists on how to minimise the cardiac effects of cancer treatment.”
Professor Ibáñez concluded: “The RESILIENCE project is breaking boundaries between different stakeholders in the cardio-oncology field, with the common goal of improving patients’ quality of life.”