- This Project has received funding from the European Union´s Horizon 2020 research and innovation programme under grant agreement No 945118
- Home
- The ProjectThe Resilience Project
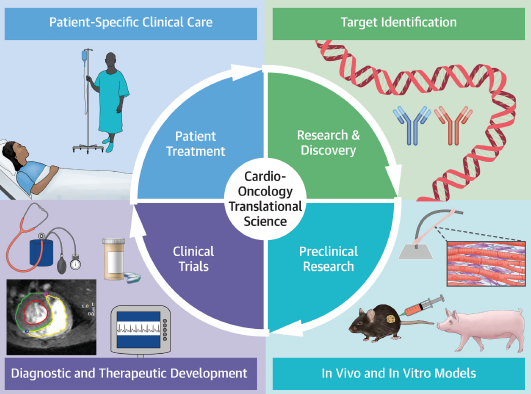
The RESILIENCE project develop a novel healthcare intervention (Remote Ischemic Conditioning, RIC) aimed at reducing the prevalence of chronic heart failure in cancer survivors.
Reduce the Heart Failure in Cancer SurvivorsAccording to the WHO, the incidence of cancer is expected to rise by about 70% over the next 20 years. Half of those diagnosed with cancer will survive for at least a decade, and this survival rate is expected to increase significantly in the future.Remote Ischemic ConditioningRemote ischemic conditioning (RIC) is a phenomenon by which brief, reversible episodes of occlusion and re-occlusion in one organ (e.g. an arm) render remote organs resistant to injury.
- Cardiac Magnetic Resonance
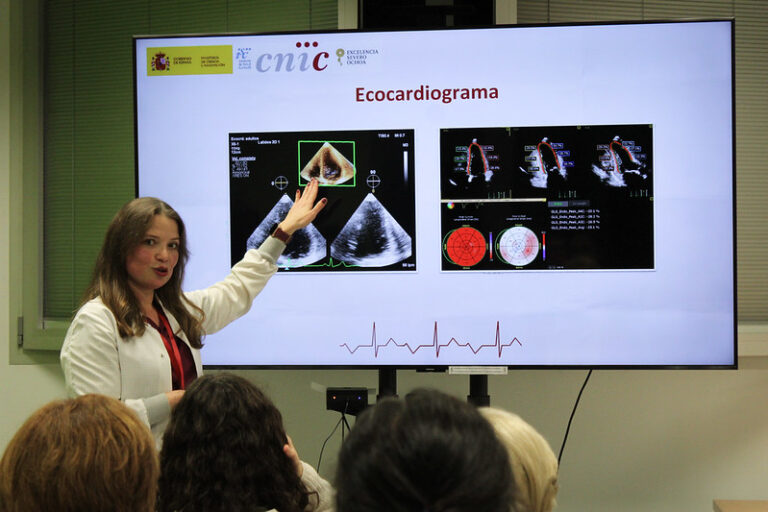
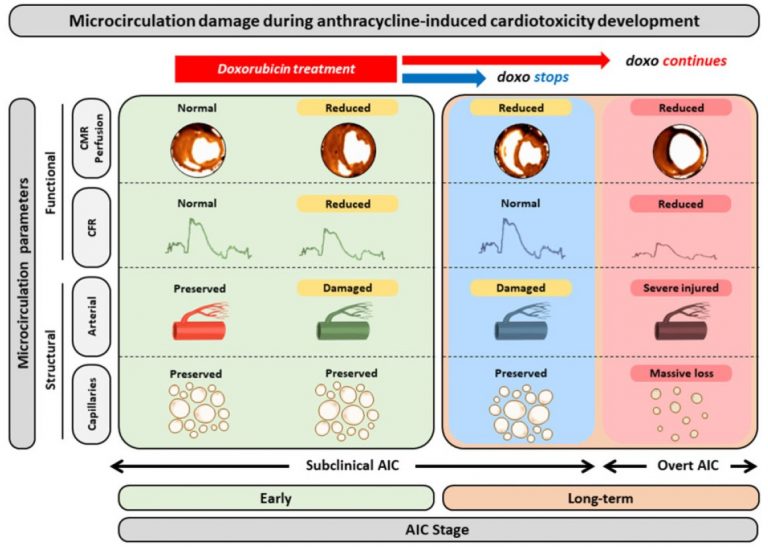
Cardiac magnetic resonance (CMR) it is a technique without radiation, which is considered the gold-standard for the evaluation of cardiac anatomy, function and even tissue composition. The RESILIENCE trial will use CMR as the main outcome measure methodology, and patients enrolled in the trial will undergo 3 scans across the duration of the study.



- Clinical Trial
Multinational, prospective, proof of concept phase II, double-blinded, sham-controlled, randomized clinical trial (RCT) to evaluate the efficacy and safety of Remote Ischaemic Conditioning (RIC) in Lymphoma patients receiving anthracyclines. Patients scheduled to undergo ≥5 chemotherapy cycles will be eligible.

- For patients
- DisseminationJuly 7 2024November 29 2023December 23 2021
- Blog