Evolution of RESILIENCE
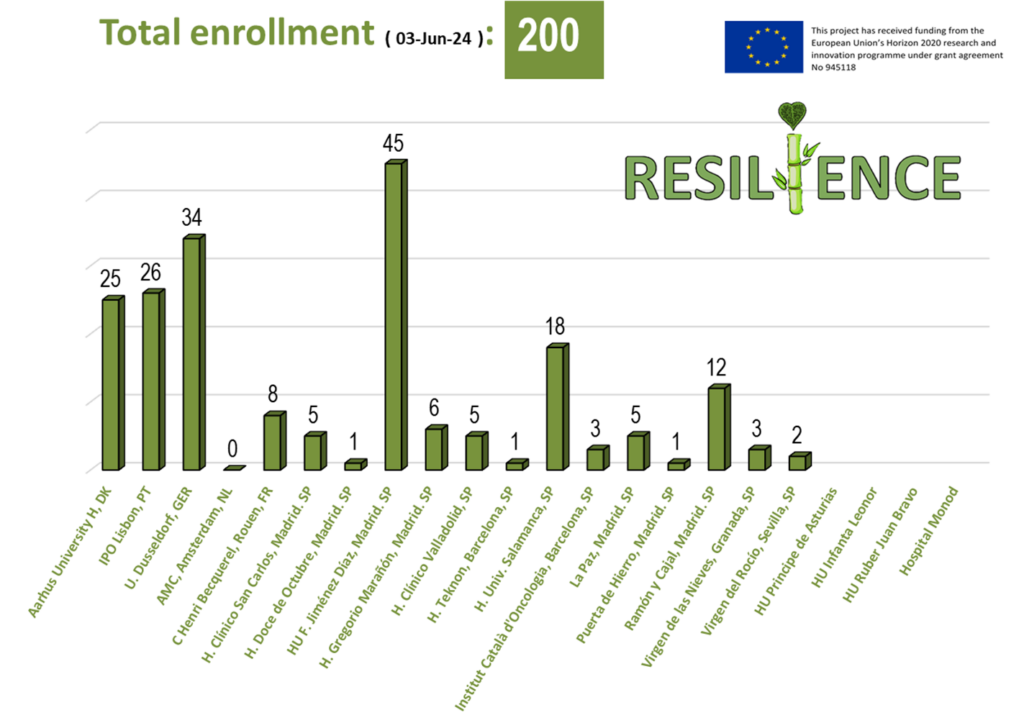
Newsletter N.10, June 2024 This Project has received funding from the European Union´s Horizon 2020…
The RESILIENCE project develop a novel healthcare intervention (Remote Ischemic Conditioning, RIC) aimed at reducing the prevalence of chronic heart failure in cancer survivors.
Remote ischemic conditioning (RIC) is a phenomenon by which brief, reversible episodes of occlusion and re-occlusion in one organ (e.g. an arm) render remote organs resistant to injury.
Cardiac magnetic resonance (CMR) it is a technique without radiation, which is considered the gold-standard for the evaluation of cardiac anatomy, function and even tissue composition. The RESILIENCE trial will use CMR as the main outcome measure methodology, and patients enrolled in the trial will undergo 3 scans across the duration of the study.
Multinational, prospective, proof of concept phase II, double-blinded, sham-controlled, randomized clinical trial (RCT) to evaluate the efficacy and safety of Remote Ischaemic Conditioning (RIC) in Lymphoma patients receiving anthracyclines. Patients scheduled to undergo ≥5 chemotherapy cycles will be eligible.

Cancer survivors are at high risk for cardiovascular complications. Anthracyclines are an extremely effective treatment against many cancer types, but they can induce cardiac toxicity resulting in chronic heart failure.
RESILIENCE Project tests a novel preventive intervention (remote ischemic conditioning) in patients at risk for anthracycline-induced cardiotoxicity. In addition, new diagnostic modalities will be used to identify anthracyclines-induced cardiotoxicity in its very early stages.
RESILIENCE counts on a multidisciplinary consortium with the active participation of patients.
Newsletter N.10, June 2024 This Project has received funding from the European Union´s Horizon 2020…

In the framework of the European Researchers’ Night and the Science and Innovation Week to…
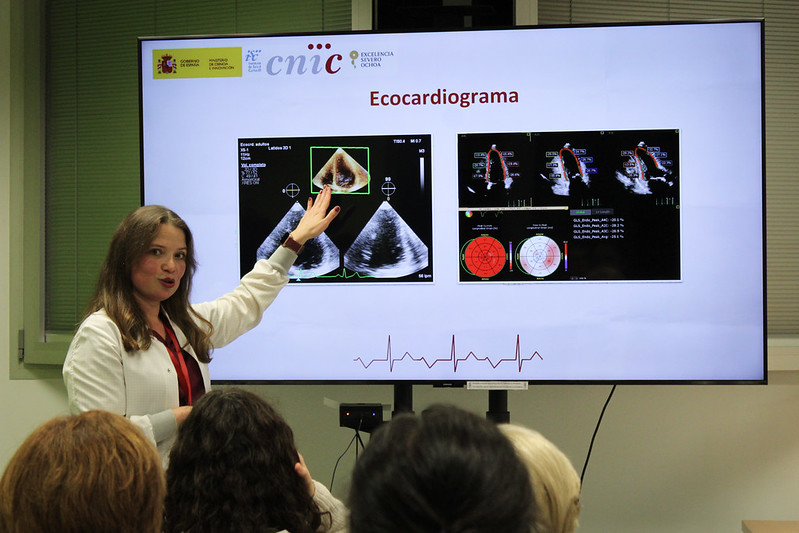
Since we started the RESILIENCE project, we have held regular encounters with our patients, and…

Due to the commemoration of the European Day for the Prevention of Cardiovascular Risk on…

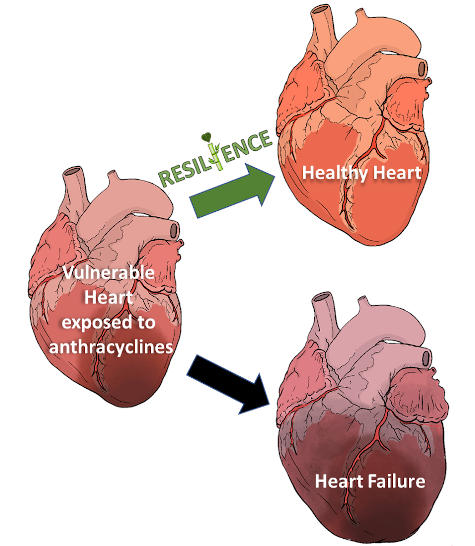
Thanks to the great scientific and medical advances, the prevalence of cancer survivors in increasing in Europe. However, cancer survivors are at high risk for cardiovascular complications. Among them, heart failure is especially worrisome. There is a strong need to reduce the burden of disability in cancer survivors and to increase their quality of life.
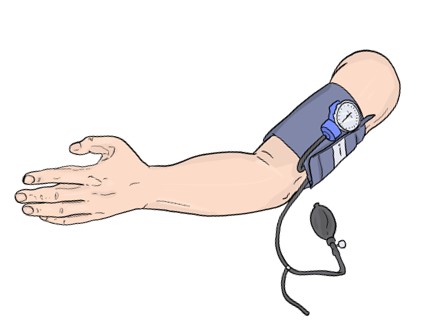
Remote Ischemic Conditioning (RIC) is a phenomenon by which brief, reversible episodes of occlusion and re-occlusion in one organ (e.g. an arm) render remote organs resistant to injury.
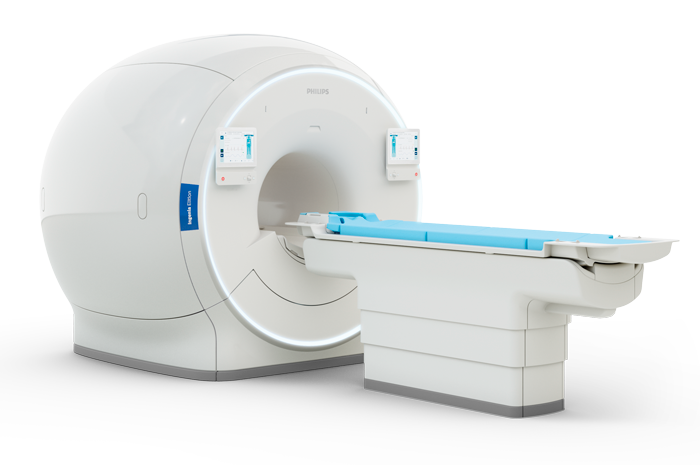
Cardiac magnetic resonance (CMR) it is a technique without radiation, which is considered the gold-standard for the evaluation of cardiac anatomy, function and even tissue composition.
Multinational, prospective, proof of concept phase II, double-blinded, sham-controlled, randomized clinical trial (RCT) to evaluate the efficacy and safety of Remote Ischaemic Conditioning (RIC) in Lymphoma patients receiving anthracyclines. Patients scheduled to undergo ≥5 chemotherapy cycles will be eligible.
Lymphoma treatment includes the use of anthracyclines within a homogeneous combination chemotherapy. Lymphomas are specially vulnerable to anthracycline-induced heart failure. Lymphoma affects similarly women and men, and it it thus ideal to study gender differences in cardiotoxicity.

Read the most recent scientific advances in the field by RESILIENCE partners.

Read the most recent newsletters, press releases, and other information about RESILIENCE in layperson´s language.

Are you a patient participating in RESILIENCE? Here you will find useful information about the details of your participation, activities specifically designed for you. You will also be able to contact RESILIENCE researchers.
Multinational, prospective, proof of concept phase II, double-blinded, sham-controlled, randomized clinical trial (RCT) to evaluate the efficacy and safety of Remote Ischemic Conditioning.
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |