To guarantee our approach to the patients participating in the RESILIENCE clinical trial, and offer them the opportunity to exchange experiences of their participation in the clinical trial in a safe space, we regularly organize round tables between them and our medical team.
In these debates the patients can share their feelings closely, providing valuable information to the project and helping us to keep its progression in a realistic way.
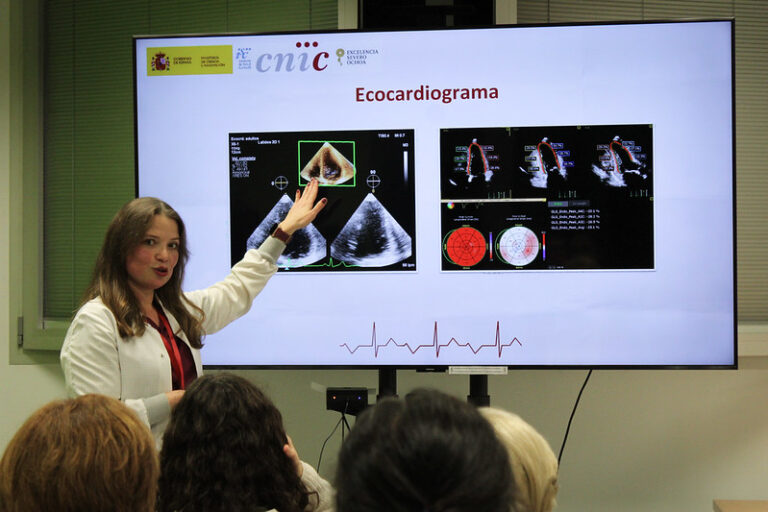
Round table with the patients
In the last meeting that we organized at the Spanish National Center for Cardiovascular Research (CNIC), one of our patients, Benito, highlighted the importance of the voluntary nature of the trial, “it is very good that it is optional and without obligation,” he commented. A crucial factor for him was the close monitoring by our medical team that patients receive. He has shared with us his concern about how the remote ischemic conditioning device protects the heart, a topic on which he has been informed by our team and helped the rest of the patients to gain knowledge about this subject.
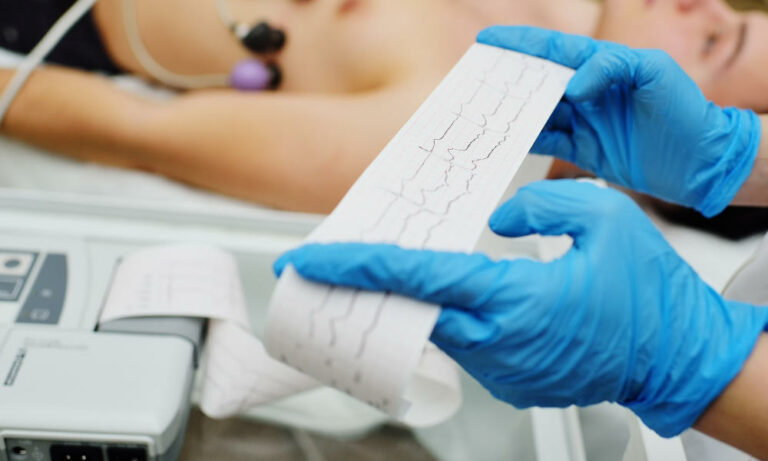
Another patient who came to this round table, María, has strongly recommended participation in the trial, describing it as “very valuable for a patient.” She has admitted that at first, she had many doubts about participating, but the possibility of trying it with the option of being able to abandon it at any time was a great relief. Close monitoring by our medical team was also of great importance to her and she confessed that she had overcome her fear of closed spaces during the cardiac magnetic resonance (CMR). The RESILIENCE trial uses CMR as the primary tool to assess outcomes, and patients undergo three CMR studies throughout the trial.
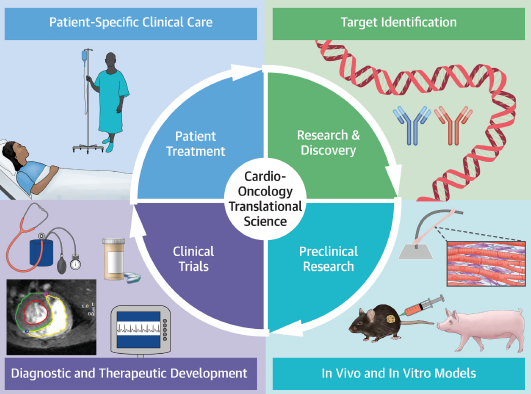
Effect of the chemotherapy on the heart
Sergio, another of the patients who attended, has shared with us positive feelings about his participation in the trial and has indicated that he had not considered the effect that chemotherapy could have on his heart. To this concern, he found motivation in the visits to the CNIC and words of encouragement about the strength of his heart. He told us that he would repeat the experience because, although he initially felt overwhelmed by the information, the process had helped him to strengthen his capacity for self-control.
“You don’t even think about what can happen to you when you receive information about your lymphoma.” They all agreed on the surprise they had experienced when asked about the effects that the treatment could have on their heart, added to the danger of searching for information on the Internet.
RESILIENCE trial
Motivation has been a common element in all of them when participating in this trial, to achieve a treatment in which we all contribute, as well as the search for a longer and quality life; stating that they would repeat and recommend the RESILIENCE trial and thanking the flexibility and kindness of the project’s medical team.
Their opinions on resonance tests are very useful to us to accompany and inform other patients about how to face these moments and their times, as well as how to try to adapt the conditions of comfort and closeness during them.
From RESILIENCE we want to thank the patients who are enrolled in the project for their participation in these meetings and share their reflections with us. Each and every one of these voices reminds us of the importance of listening to patients and adapting clinical trials to be patient-friendly and close to their needs.
The RESILIENCE clinical trial has opened a door for lymphoma patients receiving anthracyclines. This phase II, double-blind clinical trial evaluates the efficacy and safety of weekly remote ischemic conditioning.